News Archive
Weix Group Reveals New Multimetallic-Catalyzed Biaryl Synthesis
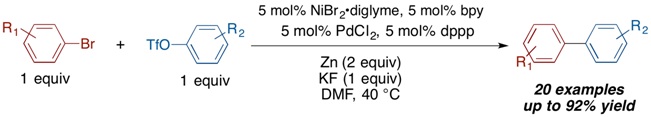
 The Weix Group recently discovered a new solution to the long-standing challenge of cross-coupling two different aryl electrophiles - a multimetallic cross-Ullman reaction. Graduate student Laura Ackerman, assisted by undergraduate Matt Lovell, developed a method to couple an aryl bromide with an aryl sulfonate ester selectively. The secret is to use a combination of two different metal catalysts, palladium and nickel, along with a simple fluoride salt. Given the abundance of the aryl starting materials and the importance of biaryls in pharmaceuticals and materials, this new reaction could find wide application.
The Weix Group recently discovered a new solution to the long-standing challenge of cross-coupling two different aryl electrophiles - a multimetallic cross-Ullman reaction. Graduate student Laura Ackerman, assisted by undergraduate Matt Lovell, developed a method to couple an aryl bromide with an aryl sulfonate ester selectively. The secret is to use a combination of two different metal catalysts, palladium and nickel, along with a simple fluoride salt. Given the abundance of the aryl starting materials and the importance of biaryls in pharmaceuticals and materials, this new reaction could find wide application.

This work was published in Nature and has also been highlighted in C&E News. Funding for this work was provided by the NIH (GM097243) and the NSF (Fellowship to Laura Ackerman).