
Joseph P. Dinnocenzo
Professor of Chemistry
PhD, Cornell University, 1983
- Office Location
- 463 Hutchison Hall
- Telephone
- (585) 275-8351
Research Overview
The vast majority of organic reactions occur via the pairwise transfer of electrons. By comparison, organic reactions that proceed via single electron transfer processes remain relatively unexplored to date. Prof. Dinnocenzo and his group are actively probing the chemistry of organic ion radicals — the products of single electron transfer.
One interesting aspect of ion radical chemistry results from their remarkably high reactivity. For example, the rearrangement of vinylcyclopropanes to cyclopentenes (e.g. 1-›2) is normally a sluggish reaction requiring high temperature, as expected based upon the Woodward-Hoffmann rules of orbital symmetry. However, we have discovered that one-electron oxidation can significantly accelerate the rearrangement. Other examples of "forbidden" pericyclic reactions that become "experimentally allowed" upon one-electron oxidation await study.
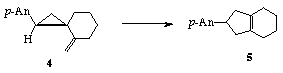
Another ion radical project involves nucleophilic substitutions on cation radicals. In contrast to conventional even-electron SN2 reactions, odd-electron SN2 reactions substitutions are dramatically faster — in fact, they are the fastest SN2 reactions discovered to date. Despite this high reactivity, the reactions can be highly selective. For example, we have shown that they occur with complete inversion of configuration. Most remarkably, we have found that odd-electron SN2 reactions show an unusual preference for substitution at hindered carbon atoms! This opens up new opportunities for their use in synthesis.
A second research theme involves an interdisciplinary, collaborative project with colleagues in Optics and at Eastman Kodak to investigate the fundamental chemical, photochemical, photophysical, polymer, and optical properties of a new class of photoresponsive polymers that undergo electron transfer-initiated ion radical chain isomerization reactions upon absorption of light. In addition to the novel amplification feature of these materials, the materials are attractive because they provide a flexible platform that can be used to design polymeric materials that respond to light by transforming any of a number of optical properties, including refractive index, absorption, fluorescence, nonlinear optical susceptibilities, as well as their spectral dependencies.
A wide variety of experimental methodologies are employed in our research, including small molecule and polymer synthesis, all of the common forms of spectroscopic characterization, stereo-chemical experiments, isotopic labeling studies, kinetic analyses (picosecond and nanosecond), photochemical techniques, fluorimetry, and time-resolved single photon counting techniques. Experimental work is frequently supplemented by state-of-the-art quantum chemical calculations.
Research Interests
- Mechanistic and physical organic chemistry
Selected Publications
- Shukla, D., Adiga, S., Ahearn, W., Dinnocenzo, J. P., Farid, S. "Chain Amplified Photochemical Fragmentation of N Alkoxypyridinium Salts: Proposed Reaction of Alkoxyl Radicals with Pyridine Bases to Give Pyridinyl Radicals," J. Org. Chem.2013, 78, 1955-1964. http://dx.doi.org/10.1021/jo301975j
- Braida, B., Hendrickx, K., Domin, D., Dinnocenzo, J. P., Hiberty, P. "Multicenter Bonding in Ditetracyanoethylene Dianion: A Simple Aromatic Picture in Terms of Three-Electron Bonds," J. Org. Chem. Theory Comput.2013, 9, 2276-2285. http://dx.doi.org/10.1021/ct400290n
- Luo, P., Dinnocenzo, J. P., Merkel, P. B., Young, R. H., Farid, S. "Biomolecular Electron Transfers that Deviate From the Sandros-Boltzmann Dependence on Free Energy: steric effect," J. Org. Chem.2012, 77, 1632-1639.
- Farid, S., Dinnocenzo, J. P., Merkel, P. B., Young, R. H., Shukla, D., Guirado, G. "Reexamination of the Rehm-Weller Data Set Reveals Electron Transfer Quenching that Follows a Sandros-Boltzmann Dependence on Free Energy," J. Am. Chem. Soc.2011, 133, 11580-11587.
- Farid, S., Dinnocenzo, J. P., Merkel, P. B., Young, R. H., Shukla, D. "Bimolecular Electron Transfers that Follow a Sandros-Boltzmann Dependence on Free Energy," J. Am. Chem. Soc.2011, 133, 4791-4801.
- Guirado, G., Haze, O., Dinnocenzo, J. P. "Generation and Characterization of 1,2 Diaryl-1,1,2,2-tetramethyldisilane Cation Radicals," J. Org. Chem.2010, 75, 3326-3331.
- Merkel, P. B., Dinnocenzo, J. P. "Low-power green-to-blue and blue-to-UV upconversion in rigid polymer films," J. Lumin.2009, 129, 303-306.
- Merkel, P.B., Luo, P., Dinnocenzo, J.P., Farid, S. "Accurate Oxidation Potentials of Benzene and Biphenyl Derivatives via Electron-Transfer Equilibria and Transient Kinetics," J. Org. Chem.2009, 74, 5163-5173.
- Merkel, P. B. and Dinnocenzo, J. P. "Experimental and Theoretical Study of Triplet Energy Transfer in Rigid Polymer Films," J. Phys. Chem. A2008, 112, 10790-10800.
- Feffar, L., Mis, M., Dinnocenzo, J. P., Farid, S., Merkel, P. B., Robello, D. R. "Quantum amplified isomerization in polymers based on triplet chain reactions," in J. Org. Chem., 2008, 73, 5683-5692.
- Gillmore, J. G., Neiser, J. D., McManus, K. A., Roh, Y., Dombrowski, G. W., Brown, T. G., Dinnocenzo, J. P., Farid, S., Robello, D. R. "Quantum Amplified Isomerization: A New Concept for Polymeric Optical Materials," Macromolecules2005, 38, 7684-7694.
- Dombrowski, G., Dinnocenzo, J. P., Zielinski, P. A., Farid, S., Wosinska, Z., Gould, I. R. "Efficient Unimolecular Deprotonation of Amine Radical Cations," J. Org. Chem2005, 70, 3791-3800.
- Robello, D. R., Dinnocenzo, J. P., Farid, S., Gillmore, J. G., Thomas, S. W., III. "Quantum Amplified Isomerization: A New Chemically Amplified Imaging System in Solid Polymers," in Chromogenic Phenomena in Polymers, Jenekhe, S. A., Kiserow, D. J. (Eds.) American Chemical Society, Washington, DC 2004, 135-146.
- Guirado, G., Fleming, C. N., Lingenfelter, T. G., Williams, M. L., Zuilhof, H., Dinnocenzo, J. P. "Nanosecond Redox Equilibrium Method for Determining Oxidation Potentials in Organic Media," J. Am. Chem. Soc.2004, 126, 14086-14094.
- Wang, Y., Luttrull, D. K., Dinnocenzo, J. P., Goodman, J. L., Farid, S., Gould, I. R. "Associative Return Electron Transfer. A Bond-Coupled Electron Transfer in the Photoreactions of Cyclopropylamines," Photochem. Photobiol. Sci.2003, 2, 1169-1176.
- Shukla, D., Liu, G., Dinnocenzo, J. P., Farid, S. "Controlling Parameters for Radical Cation Fragmentation Reactions: Origin of the Intrinsic Barrier," Can. J. Chem.2003, 81, 744-757.
- Kiau, S., Dinnocenzo, J. P., Farid, S., Goodman, J. L., Gould, I. R., Young, R. H. "Kinetics of Isomerization via Photoinduced Electron Transfer I. Spectral Analysis and Structural Reorganization of Hexamethyl Dewar Benzene Exciplexes," J. Phys. Chem. A2003, 107, 3625-3632.
