Our Projects
The PhotoROC Center will use light harvested by nanoparticles to drive the reduction of CO2 to useful compounds such as methanol. This process stores light energy in the fuel methanol. The first step in this process is energizing an electron in a light-absorbing material. That high-energy electron then can be transferred to a catalyst to provide both the energy and electrons needed to carry out the energy-storing CO2 reduction reaction. The electrons are then replenished from a biological source. These tasks will be investigated by 3 subgroups: One subgroup will focus on light harvesting, nanoparticle synthesis and the photophysics of generating energetic electrons to initiate catalysis (photons to electrons, P2E subgroup). A second subgroup will focus on homogeneous and nanoparticle catalysts for the multistep chemical cascade to convert carbon dioxide into a fuel (electrons to fuels, E2F subgroup). A third subgroup will focus on using wastewater and bacteria to provide electrons to regenerate the catalyst (biological electrons, B2E subgroup). A group of theoreticians with expertise on these topics will work with all three subgroups to provide computational support and mechanistic insight, and to identify promising catalyst leads.
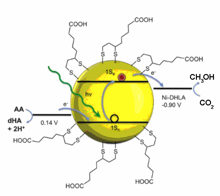
Photons to Electrons(P2E) Subgroup
Our research aims to understand all aspects of the transfer of a photoexcited electron from a semiconductor nanoparticle surface to a molecular based catalyst, a key process in any photo-catalytic cycle. Specific challenges being addressed through our interdisciplinary research program include: (1) optimizing the interactions between nanoparticle surface capping groups and molecular catalysts, (2) understanding photostability of the nanoparticles and the catalysts, (3) determining timescales for electron transfer compared with radiative and nonradiative recombination, (4) measuring the energetics of the photogenerated electron during the catalytic cycle, (5) studying assembly and transport of charge in nanoparticle films on electrode surfaces, and (6) characterizing the structure-performance relationships of nanoparticle-catalysts with novel in-situ probes. Towards these goals, we have assembled a collaborative research team with synergistic experimental and theoretical expertise whereby experiment informs theory and vice versa. Beyond fundamental scientific discoveries, we are also excited about the prospect of building highly engineered nanoparticle superlattices for catalysis and engineered solar concentration systems for delivering sunlight to chemical reactors.

Electrons to Fuels (E2F) Subgroup
The goal of this subgroup is to develop new catalysts that can combine CO2 with the electrons and protons generated in the other two groups to form methanol. This step stores the energy harvested from organic waste (B2E subgroup) and sunlight (P2E subgroup) in the strong carbon-hydrogen chemical bonds of methanol. Methanol is easily stored and the energy can be easily released by burning the methanol (current technology) or by using it in a fuel cell (new technology).
In order to accomplish this goal, the E2F group will design and study new, more affordable and active catalysts for several different routes (A-C, A+D, or E+F) as well as examine how to couple them to the P2E nanoparticle photocatalysts. The resulting catalysts will likely be useful for other challenging chemistry problems, such as the conversion of organic waste into useful chemicals. Just as importantly, illuminating how/why these reactions work will significantly improve our understanding of fundamental chemical processes.
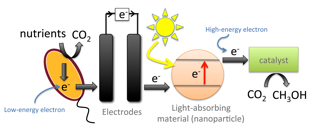
Biological Electron Donors (B2E) Subgroup
As mentioned above, during the production of methanol the electron-donating material is depleted of electrons, which need to be replenished. There are a number of ways that these electrons can be supplied, but we are taking a new approach by obtaining electrons from organic compounds commonly found in wastewater. To get the needed electrons from these compounds, we feed them to bacteria that extract and transfer electrons to extracellular substrates in a process called "extracellular electron transfer." This process allows us to use inexpensive organic materials as a source of the needed electrons for the fuel-forming reaction.

