 The selective formation of carbon-carbon and carbon-heteroatom bonds is of fundamental importance in the synthesis of pharmaceuticals, natural products and bio-active molecules for both health related research and clinical use. For example, reactions including iron-catalyzed C-C cross-coupling and olefin aminofunctionalizations have emerged as highly promising alternatives to traditional precious metal catalysis, offering improved sustainability, reduced cost and toxicity, and opportunities for novel reactivities. However, iron remains significantly underdeveloped compared to precious metal catalysts in these areas and across organic synthesis as a whole. Currently available methods only begin to address the potential of iron for catalysis in these bond forming processes and numerous challenges and areas for significant improvement remain. For example, within iron-catalyzed cross-coupling these include the current requirements for large amounts of toxic NMP co-solvent in many reactions, the lack of effective Suzuki-Miyaura cross-couplings utilizing simple boronic acids, and the need to broaden the scope of the nucleophiles and electrophiles that can be cross-coupled.
The selective formation of carbon-carbon and carbon-heteroatom bonds is of fundamental importance in the synthesis of pharmaceuticals, natural products and bio-active molecules for both health related research and clinical use. For example, reactions including iron-catalyzed C-C cross-coupling and olefin aminofunctionalizations have emerged as highly promising alternatives to traditional precious metal catalysis, offering improved sustainability, reduced cost and toxicity, and opportunities for novel reactivities. However, iron remains significantly underdeveloped compared to precious metal catalysts in these areas and across organic synthesis as a whole. Currently available methods only begin to address the potential of iron for catalysis in these bond forming processes and numerous challenges and areas for significant improvement remain. For example, within iron-catalyzed cross-coupling these include the current requirements for large amounts of toxic NMP co-solvent in many reactions, the lack of effective Suzuki-Miyaura cross-couplings utilizing simple boronic acids, and the need to broaden the scope of the nucleophiles and electrophiles that can be cross-coupled.
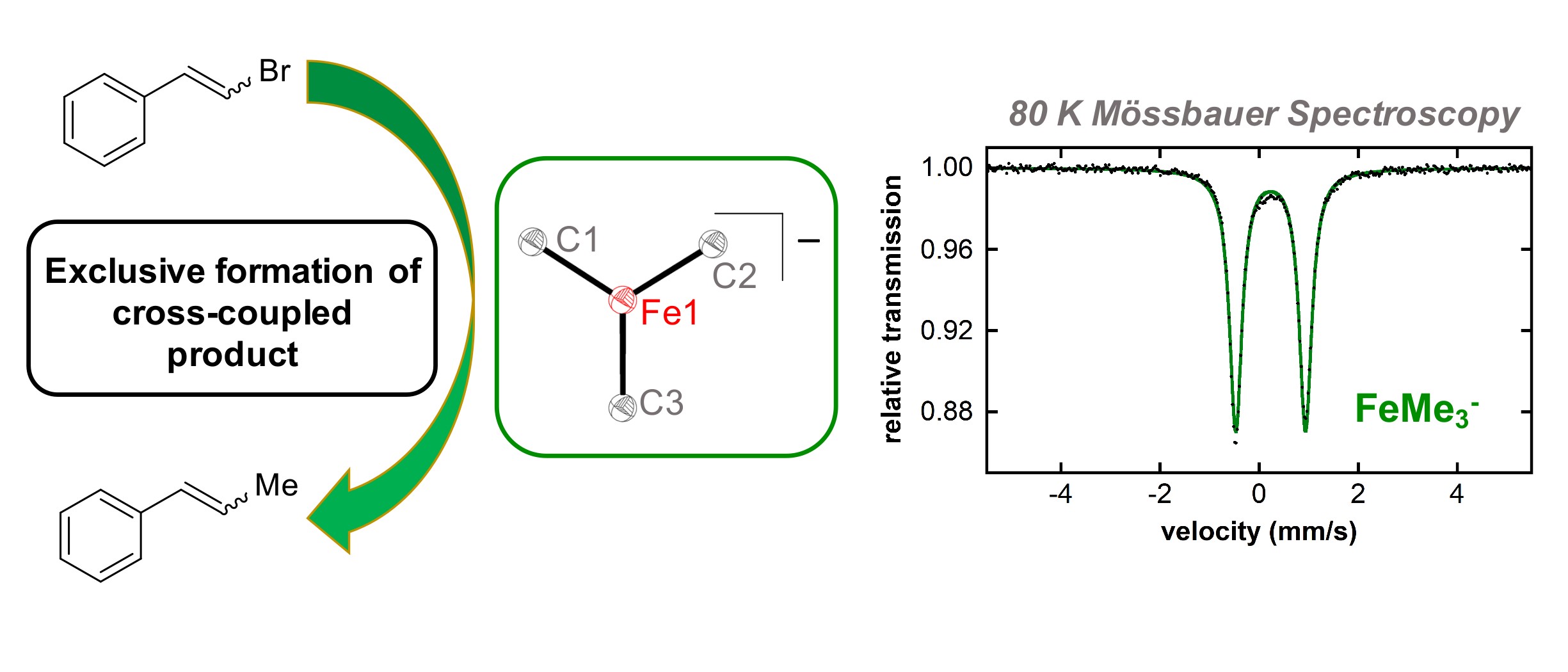
Work in the Neidig group is derived from the hypothesis that a detailed understanding of active catalyst structure, ligand and additive effects, and mechanism can provide the basis for improvements in current catalytic systems, as well as the inspiration for the development of new catalysts and methodologies that will greatly expand the scope and utility of iron in C-C and C-heteroatom bond forming reactions. So far, our group has focused on the development and application of a physical-inorganic based methodology to provide direct molecular-level insight into the fundamental chemistry underlying iron cross-coupling catalysis. This should enable rational, mechanistically driven catalyst development, the likes of which have proven widely successful in palladium chemistry. Representative contributions from our group in this area include (1) identification of the active iron species in iron-SciOPP and iron-NHC catalyzed cross-couplings including the importance of iron(II) active species; (2) determination of Kochi’s reactive iron species in simple iron salt cross-couplings and the NMP effect on iron speciation and reactivity in cross-coupling with simple ferric salts and MeMgBr; (3) elucidation of the effect of β-hydrogens on iron speciation and reactivity in cross-coupling reactions, employing simple ferric salts and alkyl Grignard reagents, (4) synthesis, characterization and reactivity of multinuclear iron-phenyl species formed upon reaction of Fe(acac)3 and PhMgBr and (5) determination of the mechanism of iron-catalyzed hydromagnesiation of styrene derivatives.
- Neate, P. G. N.; Greenhalgh M. D.; Brennessel, W. W.; Thomas, S. P.; Neidig, M. L., “TMEDA in iron-catalyzed hydromagnesiation: Iron(II)-alkyl species for controlled reduction to alkene-stabilized iron(0)” Angew. Chem. Int. Ed. 2020, 59, 17070–17076.
- Neidig, M. L.; Carpenter, S. H.; Curran, D. J.; DeMuth, J. C.; Fleischauer, V. E.; Iannuzzi, T. E.; Neate, P. G. N.; Sears, J. D.; Wolford, N. J., “Development and evolution of mechanistic understanding in iron-catalyzed cross-coupling.” Acc. Chem. Res. 2019, 52, 140-150.
- Neate, P. G. N.; Greenhalgh, M. D.; Brennessel, W. W.; Thomas, S. P.; Neidig, M. L., “Mechanism of the bis(imino)pyridine-iron-catalyzed hydromagnesiation of styrene derivatives.” J. Am. Chem. Soc. 2019, 141, 10099-10108.
- Sears, J. D.; Muñoz III, S. B.; Daifuku, S. L.; Shaps, A. A.; Carpenter, S. H.; Brennessel, W. W.; Neidig, M. L., “Effect of β-hydrogens on iron speciation in cross-couplings with simple iron salts and alkyl Grignard reagents.” Angew. Chem. Int. Ed. 2019, 58, 2769-2773.
- Sears, J. D.; Neate, P. G. N.; Neidig, M. L., “Intermediates and mechanism in iron-catalyzed cross-coupling.” J. Am. Chem. Soc. 2018, 140, 11872-11883.
- Muñoz III, S. B.; Daifuku, S. L.; Sears, J. D.; Baker, T. M.; Carpenter, S. H.; Brennessel, W. W.; Neidig, M. L., “The N-Methylpyrrolidone (NMP) effect in iron-catalyzed cross-coupling with simple ferric salts and MeMgBr.” Angew. Chem. Int. Ed. 2018, 57, 6496-6500.