Research Information
Overview
The self-assembly of peptides and proteins into cross-β amyloid structures is a defining characteristic of amyloid pathologies including Alzheimer's disease, Parkinson's disease, type 2 diabetes, and prion encephalopathies. Amyloid protein assemblies are not limited to pathological conditions, but also exist as evolutionarily conserved motifs with defined biological function. There is growing interest in exploiting peptide self-assembly phenomena for the development of novel functional structures with applications in biomedicine, energy, and materials. The long-term goals of the Nilsson group are to understand the noncovalent interactions that drive peptide self-assembly in order to facilitate novel approaches to perturb and control these processes and to exploit these interactions to enable new strategies for the noncovalent synthesis of functional and dynamic self-assembled materials. We are specifically focused on three areas of study: 1) the characterization of noncovalent interactions that give rise to cross-β fibril formation and correlation of self-assembly propensity and mechanism to pathology; our research is focused on the Alzheimer's disease amyloid-β peptide and other self-assembling peptides; 2) the development of peptides that self-assemble under the control of biologically relevant stimuli; 3) exploiting the amyloid-like self-assembly of small molecule amino acid derivatives to form hydrogel materials for applications in regenerative medicine and drug delivery. Our interests in each of these focus areas are described below.
Amyloid self-assembly and pathology
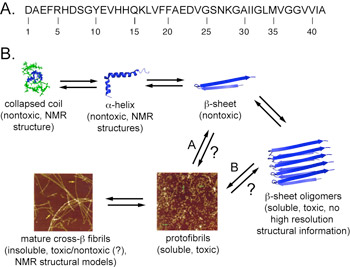
The precise mechanisms that promote self-assembly of peptides and proteins into amyloid architectures are not well understood. In addition, the relationship between amyloid self-assembly, which proceeds through various transient intermediates en route to amyloid fibrils (Figure 1), and pathology is poorly characterized. We have conducted fundamental studies that clarify how the hydrophobicity, steric profile, and aromatic character of the constituent amino acids of self-assembling peptides influence the formation higher order amyloid structures.(1-4) These studies form the basis for ongoing efforts in the Nilsson lab that focus on understanding the structure and function of prefibrillar oligomer structures of the Alzheimer's disease Aβ peptide (Figure 1). Specifically, we are developing ligands that perturb the stability and toxicity of prefibrillar oligomers and we have also identified lead compounds that are cytoprotective against Aβ-mediated toxicity at very low concentrations relative to existing compounds.
Amyloid structures found in semen have been found to play an important role in the sexual transmission of HIV.(5) These semen-derived fibrils (semen-derived enhancer of virus infection–SEVI) dramatically increase the infectivity of HIV. We have established that the cationic nature of SEVI is responsible for the observed effects on HIV infection and that interfering with virus-SEVI interactions can reduce HIV infectivity.(6, 7) This work has been funded by a Creative and Novel Ideas in HIV Research (CNIHR) Award (jointly supported by the NIH and the International AIDS Society). Future efforts will focus on establishing the mechanisms for formation of SEVI fibrils in the unusual environment of seminal fluid and on understanding the biological significance of SEVI to HIV infection. In addition, we are exploring the use of hydrogels derived from simple amphipathic self-assembling peptides as functional materials for anti-HIV microbicides. These anti-infective materials have potential as vaginal and rectal microbicides to prevent sexual transmission of HIV.
Stimulus-responsive peptide self-assembly
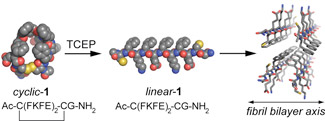
The ability to elicit peptide self-assembly in response to a biologically relevant stimulus facilitates the use of self-assembling peptides in specific microenvironments. Recently we reported the development of a reductive trigger for peptide self-assembly and subsequent hydrogelation [this work was featured as an Editor's Choice in Science 2010, 328, 669].(8) We found that cyclization of the Ac-C(FKFE)2CG-NH2 peptide sequence via intramolecular disulfide bond formation between the flanking Cys residues provides a conformational restraint that prevents the macrocyclic form of this peptide from adopting the β-sheet conformation that is required for self-assembly into fibril superstructures (Figure 2). Upon reduction of the disulfide bond, the peptide relaxes into a β-sheet conformation and immediate self-assembly into soluble, amyloid-like bilayer fibrils occurs. A reductive trigger for peptide self-assembly is sensitive to biological microenvironments. We are interested in exploring the consequence of self-assembly of foreign peptides within cells and these studies are currently ongoing.
Self-assembled hydrogels for tissue engineering
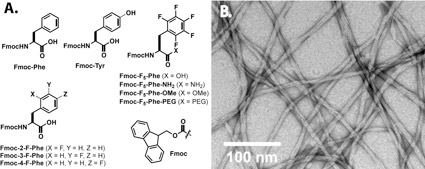
Materials that self-assemble into amyloid-like fibrils can be used to form noncovalent networks that promote hydrogelation of aqueous media in a manner similar to traditional covalent polymers.(9) Peptides and proteins that promote hydrogelation upon self-assembly have been used as materials for ex vivo tissue engineering. Ultimately, the relationship between self-assembly and emergent higher order behaviors such as hydrogelation are not well understood. In addition, peptides and proteins can be expensive to produce. As a result, we have engaged in efforts to study self-assembly/hydrogelation processes using functionalized amino acid derivatives (as opposed to lengthier peptides) in order to develop materials that can be easily and inexpensively produced and that possess the necessary properties to function as scaffolds for tissue engineering. We have conducted studies using N-functionalized phenylalanine (Phe) derivatives that explore self-assembly into fibrils and gelation phenomena in water (Figure 3).(10-14) Collectively, this work indicates that the N-terminal, side chain, and C-terminal functionality exert a significant role on self-assembly and hydrogelation of these derivatives. Minor changes (even single atom substitutions) dramatically influence both self-assembly and hydrogel formation. The fundamental reasons for this variability in hydrogelation behavior are mysterious. The objective of our future research is to address this lack in collective knowledge concerning self-assembly and hydrogelation in order to bridge the gap between empiricism and rational design in development of hydrogelators for tissue engineering.
References
- Bowerman, C. J., Ryan, D. M., Nissan, D. A., and Nilsson, B. L. (2009) The effect of increasing hydrophobicity on the self-assembly of amphipathic β-sheet peptides, Mol. BioSyst. 5, 1058–1069.
- Bowerman, C. J., Liyanage, W., Federation, A. J., and Nilsson, B. L. (2011) Tuning β-Sheet Peptide Self-Assembly and Hydrogelation Behavior by Modification of Sequence Hydrophobicity and Aromaticity, Biomacromolecules 12, 2735–2745.
- Senguen, F. T., Lee, N. R., Gu, X., Ryan, D. M., Doran, T. M., Anderson, E. A., and Nilsson, B. L. (2011) Probing Aromatic, Hydrophobic, and Steric Effects on the Self-Assembly of an Amyloid-β Fragment Peptide, Mol. BioSyst. 7, 486–496.
- Senguen, F. T., Doran, T. M., Anderson, E. A., and Nilsson, B. L. (2011) Clarifying the Influence of Core Amino Acid Hydrophobicity, Secondary Structure Propensity, and Molecular Volume on Amyloid-β 16–22 Self-Assembly, Mol. BioSyst. 7, 497–510.
- Münch, J., Rücker, E., Ständker, L., Adermann, K., Goffinet, C., Schindler, M., Wildum, S., Chinnadurai, R., Rajan, D., Specht, A., Giménez-Gallego, G., Sánchez, P. C., Fowler, D. M., Koulov, A., Kelly, J. W., Mothes, W., Grivel, J.-C., Margolis, L., Keppler, O. T., Forssmann, W.-G., and Kirchhoff, F. (2007) Semen-derived amyloid fibrils drastically enhance HIV infection, Cell 131, 1059–1071.
- Easterhoff, D., DiMaio, J. T. M., Doran, T. M., Dewhurst, S., and Nilsson, B. L. (2011) Enhancement of HIV-1 Infectivity by Simple, Self-Assembling Modular Peptides, Biophys. J. 100, 1325–1334.
- Olsen, J. S., Brown, C., Capule, C. C., Rubinshtein, M., Doran, T. D., Srivastava, R. K., Feng, C., Nilsson, B. L., Yang, J., and Dewhurst, S. (2010) Amyloid Binding Small Molecules Efficiently Block SEVI and Semen Mediated Enhancement of HIV-1 Infection, J. Biol. Chem. 285, 35488–35496.
- Bowerman, C. J., and Nilsson, B. L. (2010) A Reductive Trigger for Peptide Self-Assembly and Hydrogelation, J. Am. Chem. Soc. 132, 9526–9527.
- Ryan, D. M., and Nilsson, B. L. (2012) Self-Assembled Amino Acids and Dipeptides as Noncovalent Hydrogels for Tissue Engineering, Polym. Chem. 3, 19–33.
- Ryan, D. M., Anderson, S. B., Senguen, F. T., Youngman, R. E., and Nilsson, B. L. (2010) Self-assembly and hydrogelation promoted by F5-phenylalanine., Soft Matter 6, 475–479.
- Ryan, D. M., Anderson, S. B., and Nilsson, B. L. (2010) The influence of side-chain halogenation on the self-assembly and hydrogelation of Fmoc-phenylalanine derivatives, Soft Matter 6, 3220–3231.
- Ryan, D. M., Doran, T. M., Anderson, S. B., and Nilsson, B. L. (2011) Effect of C-terminal modification on the self-assembly and hydrogelation of Fluorinated Fmoc-Phe Derivatives, Langmuir 27, 4029–4039.
- Ryan, D. M., Doran, T. M., and Nilsson, B. L. (2011) Stabilizing self-assembled Fmoc-F5-Phe hydrogels by co-assembly with PEG-functionalized monomers, Chem. Commun. 47, 475–477.
- Ryan, D. M., Doran, T. M., and Nilsson, B. L. (2011) Complementary π-π Interactions Induce Multicomponent Coassembly into Functional Fibrils, Langmuir 27, 11145–11156.