Papers
Identifying the crucial lesion in a heat-induced developmental defect
M.A. Welte, I. Duncan, S. Lindquist (1995). "The basis for a heat-induced developmental defect: defining crucial lesions." Genes Dev. 9:2240-2250. Full Article
 Lethal heat shocks perturb a wide range of cellular processes, e.g.,
respiration, ion transport, DNA synthesis, and mRNA splicing. Which of
these changes are the cause and which are the consequences of lethal
lesions induced by elevated temperatures? To identify biologically critical
targets of heat stress, we studied a developmental defect resulting from sublethal
heat treatments. Such defects often are morphologically highly specific,
and any particular defect typically can be induced only during narrow
sensitive periods. This specificity suggests that such defects are signposts
for those biological processes most sensitive to heat damage.
Lethal heat shocks perturb a wide range of cellular processes, e.g.,
respiration, ion transport, DNA synthesis, and mRNA splicing. Which of
these changes are the cause and which are the consequences of lethal
lesions induced by elevated temperatures? To identify biologically critical
targets of heat stress, we studied a developmental defect resulting from sublethal
heat treatments. Such defects often are morphologically highly specific,
and any particular defect typically can be induced only during narrow
sensitive periods. This specificity suggests that such defects are signposts
for those biological processes most sensitive to heat damage.
When Drosophila embryos are exposed to a brief heat treatment during
cellularization, the resulting adult flies display homeotic transformations
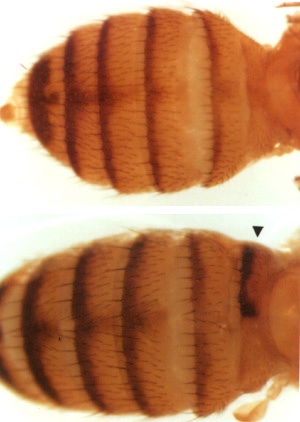
in their abdomen. In the photographs to the left, the upper panel shows
the wild-type pattern of an abdomen, the lower the defect induced after embryonic
heat treatment. Here half of the first abdominal segment (arrowhead)
shows pigmentation and bristles typical of more posterior segments.
Ian Duncan had
recognized that these heat-induced transformations mimic those caused
by certain dominant alleles of the segmentation gene fushi tarazu
(ftz ). These ftzUal alleles encode Ftz proteins that have
increased half-lives and that accumulate to abnormally high levels. In
a collaboration, we found that ftzUal mutations and the heat-induced
defect resemble each other not only in the adult phenotype, but also
by several molecular and genetic
criteria, suggesting that they are caused by the same molecular lesion,
overexpression of ftz. Indeed, heat shock blocks turnover of Ftz protein
in tissue culture cells, heat-treated embryos appear to overexpress ftz
relative to eve, and the penetrance of the heat-induced defect depends
on the dosage
of ftz. These results indicate that one of the most heat-sensitive processes
in the cell is maintaining the correct balance of regulatory proteins.
