Papers
M.A. Welte, J.M. Tetrault, R.P. Dellavalle, S. Lindquist
"A new method for manipulating transgenes: Engineering heat
tolerance in a complex multicellular organism"
Curr. Biol. 3:842-853 (1993)
When organisms are conditioned by exposure to mildly elevated temperatures, they synthesize heat-shock proteins (hsps) and acquire protection against otherwise lethal heat treatments (thermotolerance). To analyze what role Hsp70, the major heat shock protein in Drosophila, plays in thermotolerance, we developed two new techniques:
Using the yeast FLP recombinase, we created a series of transgenic
fly strains that differ only in the copy number of Hsp70. In particular,
we compared a strain carrying only the 10 endogenous Hsp70 genes ("excision
strain") to a strain that carried in addition 12 additional copies
of Hsp70 ("extra-copy strain"). We then tested if higher
levels of Hsp70 affect survival after a severe heat challenge.
Embryos were pretreated at 36°C for various lengths of time to allow
them to synthesize heat-shock proteins. Then they were challenged
with a severe heat shock, and their survival was monitored
in the hatching assay.
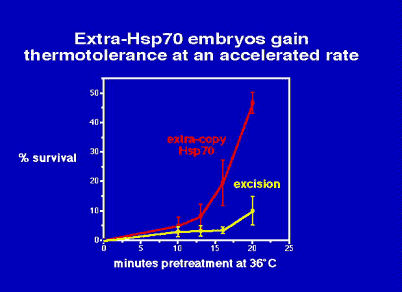
At 6 hours of embryogenesis, the extra-copy strain acquires thermotolerance
faster than the excision strain. Once Hsp70 has accumulated to a sufficient
quantity to provide as much protection as it can, both strains should survive
similarly well. Indeed, with very long pretreatments (not shown),
survival is indistinguishable.
Conclusion: In early embryos, Hsp70 is rate-limiting for the development
of thermotolerance. And manipulating the expression of a single heat-shock
protein can be sufficient to increase the thermotolerance of a multicellular
organism.
Perspective
If Hsp70 is so beneficial for stress survival, why does Drosophila
not have even more copies of Hsp70 in its genome, i.e.,
why do we not find extra-copy Hsp70 lines in nature? Apparently, increased
Hsp70 expression also carries a cost, so detrimental and
protective effects of Hsp70 need to be
balanced. Martin Feder at the University of Chicago has developed
a very active research program to unravel the function of
Hsp70 in stress tolerance. Visit
Martin
Feder's website for
exciting new findings about the benefits and costs of
Hsp70 expression.
Our transgenic lines have also been used to analyze the effect
of thermal conditioning on longevity. Heat-induced expression of Hsp70
increases lifespan at normal temperatures.
