Nucleic Acids Res 1997 Aug 15;25(16):3345-53
Department of Biology, University of Rochester, Rochester, NY 14627, USA. tip@cb.biology.rochester.edu
The GAGA transcription factor of Drosophila melanogaster is ubiquitous and plays multiple roles. Characterization of cDNA clones and detection by domain- specific antibodies has revealed that the 70-90 kDa major GAGA species are encoded by two open reading frames producing GAGA factor proteins of 519 amino acids (GAGA-519) and 581 amino acids (GAGA-581), which share a common N-terminal region that is linked to two different glutamine-rich C-termini. Purified recombinant GAGA-519 and GAGA-581 proteins can form homomeric complexes that bind specifically to a single GAGA sequence in vitro. The two GAGA isoforms also function similarly in transient transactivation assays in tissue culture cells and in chromatin remodeling experiments in vitro . Only GAGA-519 protein accumulates during the first 6 h of embryogenesis. Thereafter, both GAGA proteins are present in nearly equal amounts throughout development; in larval salivary gland nuclei they colocalize completely to specific regions along the euchromatic arms of the polytene chromosomes. Coimmunoprecipitation of GAGA-519 and GAGA-581 from crude nuclear extracts and from mixtures of purified recombinant proteins, indicates direct interactions. We suggest that homomeric complexes of GAGA-519 may function during early embryogenesis; both homomeric and heteromeric complexes of GAGA-519 and GAGA-581 may function later.
PMID: 9241251, UI: 97388557
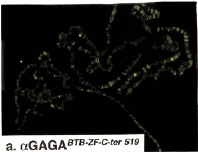
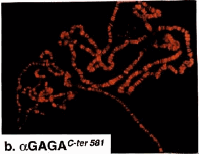
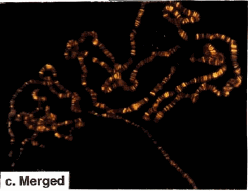
Colocalization of GAGA-519 and GAGA-581 along polytene chromosomes.
(a), (b) (c) Fluorescent micrographs of a single set of polytene chromosomes costained with affinity-purified anti-GAGABTB-ZF-C-ter 519 (green) and anti-GAGAC-ter 581 (red) antibodies.
(c) Double exposure (merged) showing regions of overlap (yellow). Note the absence of chromocenter staining (upper right).


